Question 1:
PH = 7.299 that is moderate acidaemia (less than 7.3), however we can argue that is mild as it is just 0.001 less than the value for mild acidaemia – ultimately, it is not going to change the management of the patient.
pCO2 = 52 mmHg, so we have respiratory acidosis.
Next we look at the compensation, for acute respiratory acidosis, we expect HCO3 to increase by 1 for every 10 CO2 above 40. In this case pCO2 was 12 above 40.
Accordingly, expected HCO3 should be 24 + 12 x 0.1 = 25.2. that is very close. So, this patient has appropriate respiratory compensation.
Other abnormal findings:
Cl = 108 mmol/L, that is hyperchloraemia.
Now why a health young patient with asthma develops hyperchloraemia? In mild asthmatic episode usually patients develop respiratory acidosis. The body compensate by loss of HCO3 and usually retention of chloride. Hyperchloremia might lead to deterioration of asthma and even development of NAGMA.
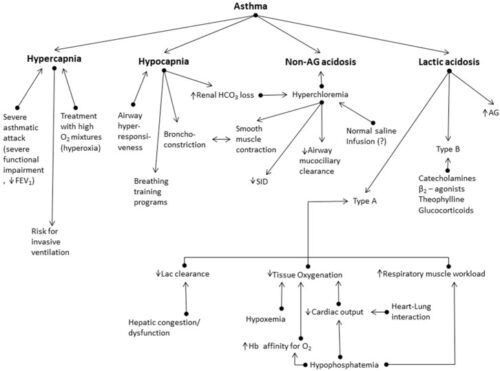
Now, how do we know that this patient doesn’t have additional NAGMA?
PH should drop by 0.08 for every 10 pCO2 above 40, accordingly, expected PH should be 7.35 – 0.08 = 7.27. This patient[s PH was higher than that, SO the patient doesn’t have additional NAGMA.
Lactate is 2.3, that is slightly elevated and that is usually due to salbutamol use.
Question 2:
The presence of acidosis in asthmatic patient indicate severe asthmatic episode. These patients should be treated promptly and aggressively. Our aim is to prevent intubation.
Treatment should start with continuous, nebulized salbutamol with intermittent Atrovent. Intravenous Steroids, Magnesium and if no response start intravenous bronchodilators (Salbutamol or Aminophylline). Consider non-invasive ventilation to those patients early.
(Basically, hit it hard and hit it fast).
References:
-
Acid-Base Disturbances in Patients with Asthma: A Literature Review and Comments on Their Pathophysiology. J Clin Med. 2019 Apr; 8(4): 563. Published online 2019 Apr 25.
