Proton Pump Inhibitor Use and Risk of Serious Infections in Young Children
Marion Lassalle, PharmD, PhD1; Mahmoud Zureik, MD, PhD1,2; Rosemary Dray-Spira, MD, PhD1
August 14, 2023 doi:10.1001/jamapediatrics.2023.2900
This cohort study examines data from French national registers for children who received treatment for gastric acid–related disorders to determine the association between use of proton pump inhibitors and serious infections in this population.
Findings
- This cohort study based on data from the French National Health Data System (SNDS) in France found that PPI exposure was associated with increased risks of serious infections overall and by infection site and pathogen.
Bottomline
- Proton pump inhibitors should not be used without a clear indication in children.
What this paper is about
- Proton pump inhibitor (PPI) use may lead to infections through alteration of the microbiota or direct action on the immune system.
- In infants, GERD may be difficult to distinguish from uncomplicated gastroesophageal reflux,1a physiological process involving spitting up, that affects up to 60% to 70% of infants at age 3 to 4 months and resolves spontaneously with standing and walking by 12 months.
- Uncomplicated gastroesophageal reflux does not require PPI treatment.3
- The study assessed the associations between PPI use and serious infections in children, overall and by infection site and pathogen.
Study Design and Population (see attached flow chart below)
- This nationwide cohort study was based on the Mother-Child EPI-MERES Register built from the French Health Data System (SNDS).
- Included all children born between January 1, 2010, and December 31, 2018, who received a treatment for gastroesophageal reflux disease or other gastric acid–related disorders, namely PPIs, histamine 2 receptor antagonists, or antacids/alginate.
- Children were followed up until admission to the hospital for serious infection, loss of follow-up, death, or December 31, 2019.
Study flowchart
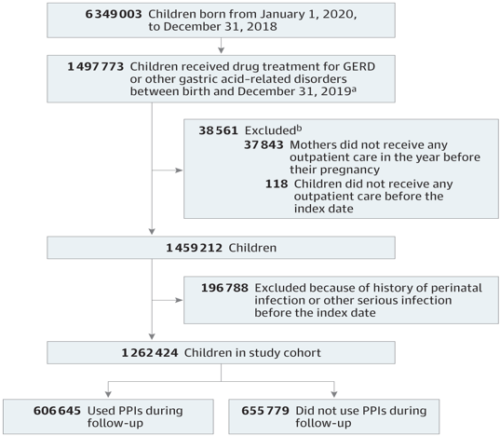 GERD indicates gastroesophageal reflux disease; PPIs, proton pump inhibitors.
GERD indicates gastroesophageal reflux disease; PPIs, proton pump inhibitors. - aThe index date is the first date any of these drugs was dispensed. bEach child may have had more than 1 exclusion criterion.
Study Outcomes and Measures
- Associations between serious infections and PPI use were estimated by adjusted hazard ratios (aHRs) and 95% CIs using Cox models.
- PPI use was introduced as a time-varying covariate
- A 30-day lag was applied to minimize reverse causality (or protopathic bias)
- Models were adjusted for sociodemographic data, pregnancy characteristics, child comorbidities, and health care utilization.
Results
- The study population comprised 1 262 424 children (median [IQR] follow-up, 3.8 years), including 606 645 who received PPI and 655 779 who did not receive PPI
- PPI exposure was associated with an increased risk of serious infections overall (aHR, 1.34; 95% CI, 1.32-1.36)
- Increased risks were also observed for infections in the digestive tract (aHR, 1.52; 95% CI, 1.48-1.55); ear, nose, and throat sphere (aHR, 1.47; 95% CI, 1.41-1.52); lower respiratory tract (aHR, 1.22; 95% CI, 1.19-1.25); kidneys or urinary tract (aHR, 1.20; 95% CI, 1.15-1.25); and nervous system (aHR, 1.31; 95% CI, 1.11-1.54) and for both bacterial (aHR, 1.56; 95% CI, 1.50-1.63) and viral infections (aHR, 1.30; 95% CI, 1.28-1.33).
Strengths of the study
- The SNDS contains comprehensive data on the entire French population, which ensures adequate power and prevents selection bias.
- The SNDS is a potent tool for identifying serious infections, with positive predictive values of 97% overall and 98% regarding the infection site
- Models were adjusted for health care utilization to minimize surveillance bias.
- Several sensitivity analyses were performed, suggesting that protopathic bias is unlikely to fully explain the associations, even for lower respiratory tract infections.
Limitations of the study
- SNDS does not provide information on indication for treatment. Therefore, they could not distinguish children experiencing GERD from those inappropriately treated for uncomplicated gastroesophageal reflux.
- Information was not available on breastfeeding or social interactions. Nevertheless, analyses were adjusted for other notable risk factors for infections, such as maternal comorbidities, prematurity, low birth weight, or cesarean delivery,
